The mesmerizing beauty of candlelight has been celebrated for thousands of years. But did you know that the sparkle of candlelight is actually created by millions of tiny, brightly burning nano-diamonds? In fact, around 1.5 million microscopic diamonds are created per second in the flame of a candle as it burns.
A challenge answered
In 2011 Professor Wuzong Zhou of the University of St. Andrews reports that a colleague from another university made the observation that no one knows, precisely, what a candle’s flame is made of. Professor Zhou became determined to solve the mystery, and said so to his colleague:
“I told him I believed science could explain everything eventually, so I decided to find out.”

The good professor, along with a student named Zixue Su, innovated a sampling technique to remove particles from the center of a candle’s flame. This was something that had never been done before. They were surprised to discover that the flame contains all four known forms of carbon.
Previous research showed that hydrocarbon molecules at the bottom of the flame were converted into carbon dioxide by the top of the flame. However, the process in between remained a mystery until Dr. Zhou’s discovery of the diamond nano-particles.
Now, in addition to graphitic and amorphous carbon, they discovered fullerenic particles and diamond nanoparticles existing in the center of the flame.

Proof positive
Using anodic aluminium oxide films as collectors, Professor Zhou, along with Zixue Su and Yang Zhang used both transmission electron microscopy and high-resolution transmission electron microscopy to publish images of soot particles from the center position of a flame.
The astonishing multitude of nano-diamond particles are seen in panels (c) and (d).
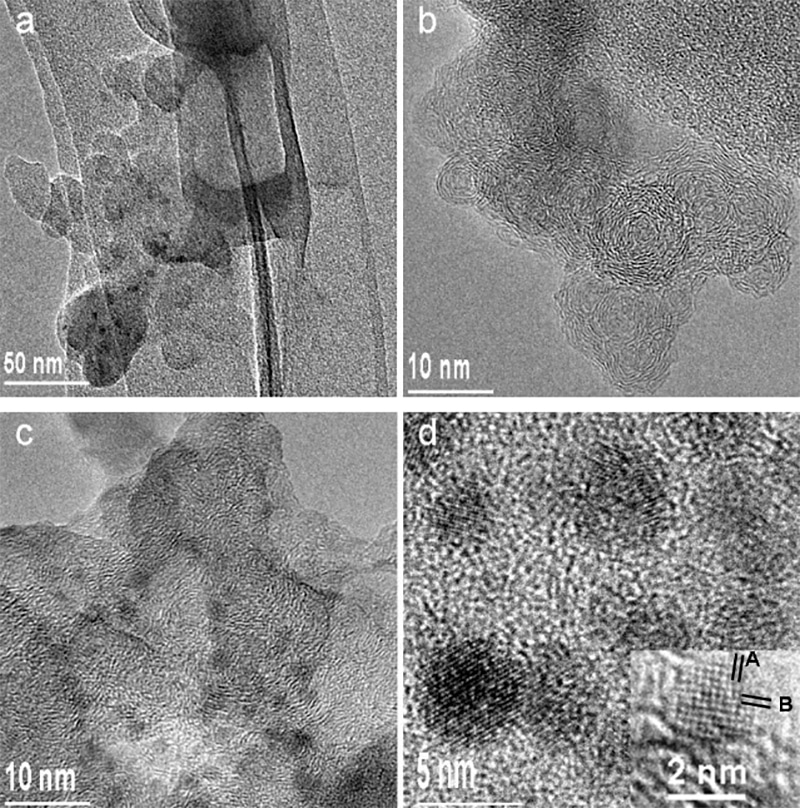
Details of this four-panel excerpt include (a) Low magnification TEM image of soot particles. (b) HRTEM image of small graphitic spheres. (c) TEM image showing many nanodiamond particles, and (d) HRTEM image of nanodiamond particles. The inset is an enlarged image of nanodiamond viewed down the [001] zone axis. The d-spacings of A and B are about 0.187 and 0.185 nm respectively, and can be indexed to (200) and (020) of the unit cell of carbon. The corresponding interplane angle is 92º.
A fleeting moment
Unfortunately there’s no way of extracting the diamond particles, since they are burned away in the process, but the discovery was key to our understanding of why candlelight takes on its singular, sparkling, mesmerizing appearance.

The famous scientist Michael Faraday, while giving his celebrated lectures on “The Chemical History of a Candle,” said in an 1860 address –
You have the glittering beauty of gold and silver, and the still higher lustre of jewels, like the ruby and diamond; but none of these rival the brilliancy and beauty of flame. What diamond can shine like flame?
And now we know, Professor Faraday. Diamonds are the flame.




